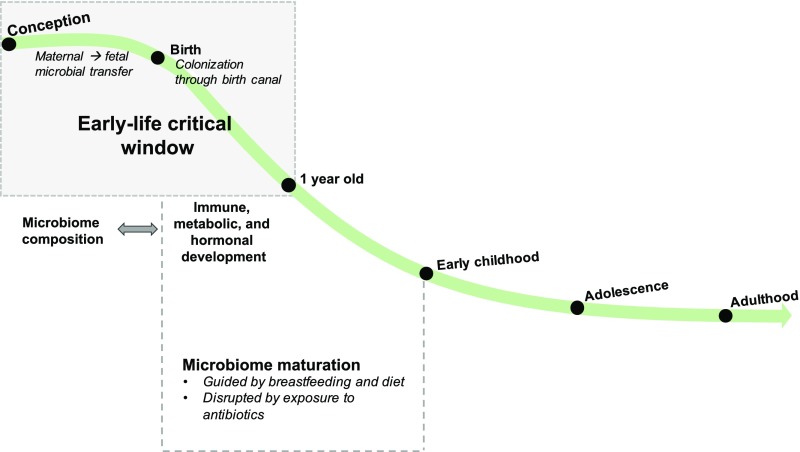
- Microbial colonization of the newborn is a pivotal process that affects later-life health.[1]
- Early-life transient dysbiosis has long-lasting effects on human health, suggesting a role of the microbiome in the Developmental Origins of Health and Disease (DOHaD).[1:1]
- Variations in the composition and functional potential of the early-life microbiome are the result of lifestyle factors, such as mode of birth, breastfeeding, diet, and antibiotic usage.[1:2]
- Variations in the composition of the early-life microbiome have been associated with specific disease outcomes, such as asthma, obesity, and neurodevelopmental disorders.[1:3]
- Variations in the microbial intrauterine environment may predispose neonates to specific health outcomes later in life.[1:4]
Early-Life Development of the Microbiome
-
The human microbiota is a composite organism, composed of 10 trillion to 100 trillion microbial cells (bacteria, archaea, and microbial eukaryotes) and viruses.[1:6]
-
The terms “microbiota” and “microbiome” are descriptive of the microbial composition and genomic catalog, respectively, but they are used interchangeably within this research field.[1:7]
-
Gut bacteria became detectable using molecular methods by 16 h after birth.[^2]
Maternal-to-Fetal Exchange of Microbes In-utero
- Until recently, the intrauterine environment was perceived as sterile. However, nonpathogenic bacteria have since been detected by molecular techniques in the amniotic fluid and placentas of healthy infants, suggesting a maternal-to-fetal exchange of microbes.[1:8]
- Through comparisons of the amniotic, placental, and meconium microbiotas, it has been reported that the meconium microbiota of infants delivered via cesarean delivery shares ∼55% of its bacterial taxa with the placenta and amniotic fluid microbiotas.[1:9]
- The prenatal maternal microbiome may also modulate the infant immune system.[1:10]
- For example, gestation-only colonization with Escherichia coli HA107 was reported to modify the intestinal mucosal innate immune system and transcriptome of the offspring.[1:11]
Placental Microbiome
-
Variations in the placental microbiome composition have been associated with maternal pregnancy-related (stress and gestational diabetes) and neonatal health outcomes (birth weight, preterm birth).[1:12]
-
The placental microbiome of infants born preterm was also reported to differ in composition according to gestational weight gain, suggesting that this bacterial consortium may mediate fetal development depending on the health status of the mother.[1:13]
-
Isolation of bacteria from the placenta is often associated with a pathophysiological state, which threatens the health of the mother and child.[1:14]
-
It is possible that the placenta does not harbor any viable bacteria but rather is composed of phagocytosed microbial by-products or cell wall components.[1:15]
-
The lack of viable bacteria does not negate the capacity of the placental microbiome to modulate fetal development because interactions with pathogen-associated molecular patterns may still regulate cell differentiation and proliferation.[1:16]
-
Upon implementation of stringent validation strategies for placental metagenomic analyses, the placental microbiome might be referenced as a biomarker for maternal and fetal health and disease.[1:17]
Vaginal Birth
Postnatal bacterial colonization of the infant begins during birth, at which time neonates are exposed to the maternal fecal and vaginal microbiotas.[1:18]
-
Within 24 hours of delivery, the microbiotas across various body sites (oral, skin, meconium, etc) of cesarean delivered infants are initially populated with bacteria residing on the mother’s skin (eg, Staphylococcus spp.), whereas vaginally delivered infants are populated with typical vaginal bacteria (eg, Prevotella, Atopobium spp.).[1:19]
- This finding may be specific to the neonatal gut microbiome. In another study of 81 mother and infant dyads, the microbiomes of other body sites (nares, skin, etc) from infants delivered vaginally revealed a bimodal pattern of maternal origin, populated by both the mothers’ vaginal and skin bacteria rather than by one or the other. However, it is suggested that the microbiotas of infants are homogeneously distributed across body sites (eg, meconium, skin, nares, etc) immediately after birth.[1:20]
-
Profiling of the neonatal intestinal microbiome immediately after birth and up to age 2 years suggests that birth mode can result in prolonged infant gut microbial dysbiosis.[1:21]
-
In a study of 43 mother and infant dyads, infants delivered via cesarean delivery exhibited increased phylogenetic diversity immediately after birth. However, after 1 month of age, the phylogenetic diversity of infants delivered via cesarean delivery declined below that of vaginally delivered infants.[1:22]
Phylogenetic diversity (PD) is a measure of biodiversity that considers the evolutionary relationships between species, rather than just the number of different species. It quantifies the amount of unique evolutionary history represented by a group of species, often visualized on a phylogenetic tree. Essentially, it reflects how different species are from each other in terms of their evolutionary lineage.
Vaginal Seeding
- After swabbing neonates with maternal vaginal fluids within 2 minutes of birth, the authors report partial restoration of the microbiota of infants delivered via cesarean delivery to that of vaginally delivered infants.[1:23]
The influence of breastfeeding in the first year of life
-
As the neonate grows, the homogeneous microbiome populating his or her body diverges into microbe-specific body niches.[1:24]
-
Driven in large part by breastfeeding and infant diet, the human gut microbiome continues to mature until the child reaches 2 to 3 years of age, after which its composition stabilizes.[1:25]
-
Breastfeeding seeds the infant gut microbiome through contact with maternal areolar and breast milk microbes and provides key energy sources for many bacteria (human milk oligosaccharides).[1:26]
- In a study of 107 mother and infant dyads, infants who were breastfed during the first 30 to 40 days of life received a mean of ∼28% of their bacteria from breast milk and ∼10% from maternal areolar skin.[1:27]
- There also seems to be a dose-dependent association between the infant gut microbiome composition and the proportion of daily breastfeeding.[1:28]
-
There is a clear compositional distinction between breastfed and formula-fed infants, with breastfed infants being populated with higher proportions of Bifidobacteria and Lactobacillus spp. and formula-fed infants being populated with a greater prevalence of clostridiales and proteobacteria.[1:29]
-
Formula-fed infants exhibit decreased diversity and bacterial richness even after the first year of life (12–24 months of age).[1:30]
-
In a study of 30 preterm infants, the effect of breastfeeding (versus formula feeding) was also reported to mask the influence of birth weight on the infant microbiome, highlighting breastfeeding as being potentially protective (at least with regard to the infant microbiome) against a traditional DOHaD risk factor.[1:31]
-
Formula feeding has been associated with an increased risk of various hyperinflammatory and immune-mediated diseases.[1:32]
-
Researchers of a recent epidemiologic study reported that breastfeeding protects against wheezing during the first year of life among infants born to mothers with asthma.[1:33]
Gestation-Associated Maternal Diet Shapes the Developing Infant Microbiome
-
Recent evidence has been used to support a significant role of gestation-associated maternal diet in shaping the microbiome of infancy. A maternal high-fat diet during pregnancy and breastfeeding was reported to induce dysbiosis in the offspring microbiome of Japanese macaques. These maternal diet–induced microbial variations persisted in juvenile macaques.44 In addition, a postweaning, low-fat control diet was unable to correct this maternal high-fat diet–induced dysbiosis.[1:34]
-
In a prospective cohort of 26 mother and infant dyads, a high-fat maternal gestational diet was associated with distinct variations in the neonatal gut microbial composition (meconium), which persisted to 4 to 6 weeks of age.[1:35]
-
In a mouse model, a maternal high-fiber diet was associated with increased short-chain fatty acid (SCFA) production in the offspring.[1:36]
- Emphasizing the immune-modulatory capacity of the maternal diet–driven microbiome during pregnancy, higher frequencies of thymic T-regulatory cells were also found in these pups.[1:37]
-
Demonstrating the effect of maternal diet on the functional capacity of the infant microbiome, piglets born of sows that were fed a western diet (high-energy, high-fat, fructose-based diet) during pregnancy displayed decreased SCFA production.[1:38]
Antibiotics Alter Infant Colonization and Decrease Microbiota Maturation
Title: The Role of the Microbiome in the Developmental Origins of Health and Disease
Publication: American Acadamy of Pediatrics: Pediatrics
Institution(s): Department of Epidemiology, Fielding School of Public Health, University of California, Los Angeles, Los Angeles, California
Archive: archive ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎ ↩︎